|
| |
|
|
| Article Published: Saturday, May 15, 2004 - 2:15:41 AM EST | ||||||||
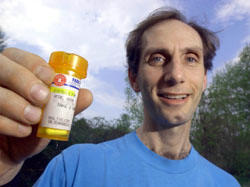
WESTMINSTER WEST -- The treatments were not working and Kevin Vetre was running out of options. It took Vetre, 39, almost two years to accept that he had multiple sclerosis. Even after his face went numb and his eyesight worsened -- two signs of the degenerative disease -- it took a conclusive MRI to convince him that the tissue around his nerve fibers was progressively deteriorating. He tried alternative medicines, and either they helped or the disease went into remission. One of the frustrating traits of the disease is that it comes and goes without warning or understanding. He managed the best he could, and for a while he believed the doctors had been wrong. But when he had to take a month off from work because he couldn't stand without leaning against a wall, he was scared enough to switch to the heavy medicine. He tried Betaseron, which he needed to inject into his body fat every other day. The drug gave him flu-like symptoms and didn't seem to halt the MS. Doctors wanted him to try a chemotherapy agent which they said could affect his heart. He refused. "The heart is the one thing I have that's working," Vetre said recently. "They may come up with a cure, and I don't want to have a bad heart." He continued: "I was hanging around my house, wheeling around an IV and I thought, this is it. I'm going to have to be a home guy. I'm going to be handicapped, and that is a hard thing to face." When an old friend called and told him that she had heard about an untested drug treatment that was showing postive results with other MS patients, he was skeptical. But he decided to investigate it on the Internet. He found out that the U.S. Food and Drug Administration ap-proved the drug, naltrexone, in 1984 as a treatment for heroin addiction. In a 50 milligram dose, it blocks the opioid receptors that heroin acts on in the brain. The side effects, however, include insomnia, depression and irritability. By 1985 most doctors had stopped prescribing it. Dr. Bernard Bihari, a Manhattan physician who was working with heroin addicts and AIDS patients, began looking into alternative uses for the drug. Bihari and his colleagues understood that large doses of naltrexone blocked the reception of endorphins in the brain. In the late 1980s, when the group was working with the drug, research was coming out showing the important effects that endorphins have on the immune system. Other tests helped doctors understand that the pituitary gland and the adrenal gland produce endorphins in the middle of the night. Bihari's group found that when a patient was given a very small dose of naltrexone just before bedtime the brain was tricked into thinking it needed to produce extra endorphin. They found the levels more than doubled by morning. "It is clear the way the body works. If a normal function is blocked the body has clever ways to get around it," Bihari said in a phone interview from his office. "The body could not overcome the high dose, but we found that the very small dose could jumpstart the development of endorphins." His group focused on AIDS patients. He said he stumbled on the benefits to people with MS when a friend of his daughter started showing signs of the disease. Bihari gave her a 3 mg dose and she showed almost instant improvements. Vetre made his way through the low-dose naltrexone Web site. "I was definitely interested," he said. Vetre went to his family doctor with information on the drug and the doctor wrote a prescription. His doctor, who did not want to be mentioned in this article, prescribed naltrexone for off-label use, which means he prescribed it for a use that has not been approved by the FDA. "There are many ways we use medicine off-label," the doctor said. "We have to be very careful, because if something bad happens there is no protection from the FDA. But if the FDA approved it in 50 milligram doses, I figured that a 3 milligram dose would be safe." Vetre could not find a local supply and he got an address off the Web site to a pharmacy in Manhattan that could supply the low dose naltrexone. When he was taking the injections, it cost him more than $1,000 a month. The new pills cost $32 for a month's supply. His insurance covers half that charge. The capsules arrive on his porch in a small envelope. In the fall of 2003, Vetre began experimenting with the new drug. He started at 3 milligrams and when that didn't take care of his physical attacks he increased it to 4 milligrams. But that, too, failed to help him. It was after he forgot to take a pill one night that he noticed he felt better on the second day. He settled on a 1.5 milligram dose. "It is pretty amazing what it does," Vetre said. "I wake up in the morning with energy. Last year, I was taking time off work, my body shook and I couldn't stand without supporting myself. Now I'm running a little and teaching. It's unbelievable." There is no cure for MS, but the FDA has approved six drugs that can slow the progression down in some people. Neither the FDA, nor the National Multiple Sclerosis Society, recognize naltrexone as a safe and effective treatment for the disease. Experts involved with MS research say that naltrexone's ability to boost the immune system goes against the accepted understanding of what might help patients. Doctors believe that an overactive immune system attacks the myelin, the fatty tissue that protects nerve fibers. All of the approved treatments inhibit the overactive immune system. "There is no published data. There has been some anecdotal evidence but really very little has been done to study how naltrexone affects MS," said Dr. Patricia O'Looney, director of the bio-medicine research program at the National Multiple Sclerosis Society. One of the society's major roles is to fund research, O'Looney said. While she has been getting calls on naltrexone, she would not recommend the drug for MS patients. Bihari said that he has contacted the society. He said they offered $25,000 which is a fraction of the millions of dollars he says he needs to conduct an adequate study. "The problem for the MS Society is that their funding comes from the six drug companies that make the six drugs that are licensed." Since the FDA has already approved naltrexone the perception exists that little money could be made by approving it as a treatment for MS, though Bihari says he owns a number of patents on the low dose and he thinks companies could see profits. He also said that the drug works in ways that baffle doctors and researchers. Modern drugs, he explains, attack cancer cells or viruses. Low dose naltrexone is unusual in that it enhances the body's defense to disease. Bihari is treating 395 MS patients with low dose naltrexone. He said a survey of around 100 pharmacies across North America showed almost 16,000 prescriptions for low dose naltrexone being used to treat the disease. He said unscientific research shows a 98 percent rate of stabilization in MS patients. "The bottom line is it works," he said. Vetre is going for an MRI this summer. Whether the MS society or the FDA approves naltrexone or not, he plans to continue his treatment. "This has completely changed the effects of the illness, and I have no reason to stop taking it. Every once in a while, I get sad that I am much more limited than I used to be, but I seem to have hit a spot where I am better," Vetre said. "When I was doing the injections, you hoped something was happening behind the scenes. But with this, you feel better. Your body tells you that you are feeling good and I haven't felt this good in a long time." Dr. Bihari answers calls at (212) 929-4196. On the Web: http://www.lowdosenaltrexone.org/ . | ||||||||
Copyright ©1999-2004 New England Newspapers, Inc.,
a member of
MediaNews Group, Inc.
| Associated Press text, photo, graphic, audio and/or video material shall not be published, broadcast, rewritten for broadcast or publication or redistributed directly or indirectly in any medium. Neither these AP Materials nor any portion thereof may be stored in a computer except for personal and non-commercial use. The AP will not be held liable for any delays, inaccuracies, errors or omissions therefrom or in the transmission or delivery of all or any part thereof or for any damages arising from any of the foregoing. |